Venkatasai is a leading innovator in the field of pharmaceutical reference standards, offering high-quality Reference Standards of Abacavir, including both pharmacopeial and non-pharmacopeial impurities, metabolites, stable isotope-labeled compounds, and nitrosamines (N-NO products). Our Abacavir impurity reference standards play a vital role in pharmaceutical research, supporting product development, ANDA and DMF filings, quality control (QC), method validation, and stability studies. They are also instrumental in identifying unknown impurities and evaluating genotoxic potential. All Abacavir-related products are thoroughly characterized and supplied with comprehensive Certificates of Analysis (COA) and analytical data that comply with regulatory standards. EP/USP traceable standards can also be provided upon request. Furthermore, all supplied products undergo periodic re-testing to ensure continued quality and reliability.
X
Venkat Sai Life Science
x
- Upcoming Expo
Phenylbutazone Related Products
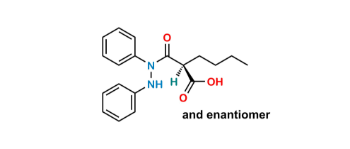
CAT No: VS-P039000
CAS No: 50-33-9
Mol.F.: C19H20N2O2
Mol.Wt.: 308.4
Status: Custom Synthesis
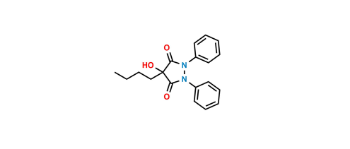
CAT No: VS-P039001
CAS No: 3583-64-0
Mol.F.: C19H22N2O3
Mol.Wt.: 326.4
Status: In Stock
CAT No: VS-P039002
CAS No: 16860-43-8
Mol.F.: C19H20N2O3
Mol.Wt.: 324.4
Status: Custom Synthesis
CAT No: VS-P039003
CAS No: 122-66-7
Mol.F.: C12H12N2
Mol.Wt.: 184.2
Status: Custom Synthesis
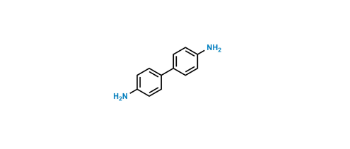
CAT No: VS-P039004
CAS No: 92-87-5
Mol.F.: C12H12N2
Mol.Wt.: 184.2
Status: Custom Synthesis
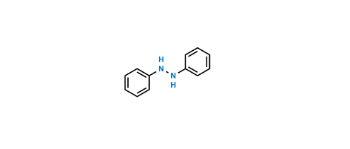
CAT No: VS-P039005
CAS No: 103-33-3
Mol.F.: C12H10N2
Mol.Wt.: 182.22
Status: Custom Synthesis